Search results
Search for "molecular switch" in Full Text gives 22 result(s) in Beilstein Journal of Organic Chemistry.
Switchable molecular tweezers: design and applications
Beilstein J. Org. Chem. 2024, 20, 504–539, doi:10.3762/bjoc.20.45

Using multiple self-sorting for switching functions in discrete multicomponent systems
Beilstein J. Org. Chem. 2020, 16, 2831–2853, doi:10.3762/bjoc.16.233

- . An astounding modus operandi of a switchable catalytic system was realized based on information processing. The switchable system actually did not rely on a molecular switch in different toggling states, but on a smart seven-component mixture that reversibly regulated two diverse catalytic ON/OFF
Easy access to a carbohydrate-based template for stimuli-responsive surfactants
Beilstein J. Org. Chem. 2020, 16, 2788–2794, doi:10.3762/bjoc.16.229
- surfactant with amphiphilic properties induced by the presence of Zn2+ ions (Figure 1b). In order to synthesize a glucopyranose-based molecular switch, a 2,4- or 3,6-functional group pattern is needed as these positions reside cis on the ring, making them pointing in the same direction of the 1C4
Thermodynamic and electrochemical study of tailor-made crown ethers for redox-switchable (pseudo)rotaxanes
Beilstein J. Org. Chem. 2020, 16, 2576–2588, doi:10.3762/bjoc.16.209

- and the redox unit, TTFC8 offers the best compromise of sufficiently high binding constants combined with sufficient Coulomb repulsion between the oxidized TTF and the ammonium ion to construct a molecular switch [35]. This trend can directly be translated to the smaller exTTFC7 and TTFC7, which
Photocontrolled DNA minor groove interactions of imidazole/pyrrole polyamides
Beilstein J. Org. Chem. 2020, 16, 60–70, doi:10.3762/bjoc.16.8
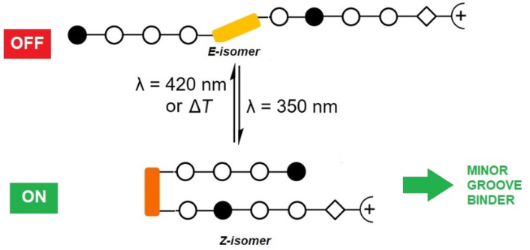
- (Py/Im) and TA (Hp/Py). However, N-methylhydroxypyrrole (Hp) is neither easy to synthesize nor sufficiently stable. In practice, therefore, Py/Py is used to address both AT and TA. Along this line, it is attractive to incorporate a molecular switch for the selective activation of an initially inactive
Experimental and computational electrochemistry of quinazolinespirohexadienone molecular switches – differential electrochromic vs photochromic behavior
Beilstein J. Org. Chem. 2019, 15, 2473–2485, doi:10.3762/bjoc.15.240
- anion rather than a photoexcited state. The reduction of the molecular switch necessary for electrochromism is in a sense catalytic: the rearranged product is reoxidized to a neutral LW isomer, which reverts thermally to SW upon standing, just as it does when the LW is generated photochemically. The
Ultrafast processes triggered by one- and two-photon excitation of a photochromic and luminescent hydrazone
Beilstein J. Org. Chem. 2019, 15, 2438–2446, doi:10.3762/bjoc.15.236

- absorption cross-section of the molecule. Keywords: hydrazone; molecular switch; pump-probe spectroscopy; time-resolved fluorescence; Introduction Molecular switches are systems that are able to rapidly respond to an external stimulus, which can be of chemical or physical nature, through a variation of
An azobenzene container showing a definite folding – synthesis and structural investigation
Beilstein J. Org. Chem. 2019, 15, 1534–1544, doi:10.3762/bjoc.15.156
- the high dispersion energy in the compact cis,cis-isomer. Keywords: azobenzene; macrocycles; molecular switch; Introduction In supramolecular chemistry rigid scaffolds are required to arrange different recognition units in predefined distances and spatial orientation to each other [1]. One example
Diazocine-functionalized TATA platforms
Beilstein J. Org. Chem. 2019, 15, 1485–1490, doi:10.3762/bjoc.15.150

- ordered monolayers on Au(111) surfaces. Keywords: cis–trans isomerization; diazocine; molecular switch; photochrome; self-assembled monolayers; TATA platform; Introduction Catalysts increase chemical reaction rates by lowering the activation energies and thus create more favorable reaction pathways [1
Tetrathiafulvalene – a redox-switchable building block to control motion in mechanically interlocked molecules
Beilstein J. Org. Chem. 2018, 14, 2163–2185, doi:10.3762/bjoc.14.190

- introduction to readers new to the field of TTF-switchable MIMs. Review 1. Tetrathiafulvalene – an (almost) perfect molecular switch Whereas inorganic chemists are used to commonly handle metal-based compounds in different oxidation states, only a small selection of organic molecules [22] can be reversibly
- molecular switch. A first one-electron oxidation [23] converts neutral TTF (1) into the radical-cationic species 1●+ (Figure 1). The TTF radical cation is one of the rare organic radicals that are long-term stable and even isolable. A second oxidation step yields the dication 12+. Both redox-transitions are
- dihydroxynaphthalene station. Chemical reduction with ascorbic acid or Na2S2O5 restores the initial spectroscopic properties and the initial co-conformation of the catenane. A tristable molecular switch based on a [2]catenane with three different stations was created by Wasielewski, Stoddart, and co-workers in 2015
A pyridinium/anilinium [2]catenane that operates as an acid–base driven optical switch
Beilstein J. Org. Chem. 2018, 14, 1908–1916, doi:10.3762/bjoc.14.165

- molecule; molecular switch; Introduction [2]Rotaxane molecular shuttles [1][2][3][4][5] are the dynamic building blocks of a wide variety of molecular switches [6][7][8][9] and a number of sophisticated molecular machines that operate away from equilibrium [10][11][12][13][14][15]. We have previously
- (pyridinium)ethane recognition sites linked by terphenyl spacer groups [17] (Figure 2). It was thus of interest to design and build these two different recognition sites (benzylanilinium and bis(pyridinium)ethane) into an analogous circumrotational [2]catenane molecular switch to compare to the linear [2
London dispersion as important factor for the stabilization of (Z)-azobenzenes in the presence of hydrogen bonding
Beilstein J. Org. Chem. 2018, 14, 1238–1243, doi:10.3762/bjoc.14.106

- applications in recent years. This molecular switch has been utilized inter alia in the rising field of photopharmacology [2][3], the manipulation of biomolecular processes [4][5][6] as well as in molecular machinery [7][8] and materials science [9][10][11]. Azobenzenes are highly stable, easily synthesized
Synthesis and metal binding properties of N-alkylcarboxyspiropyrans
Beilstein J. Org. Chem. 2017, 13, 1542–1550, doi:10.3762/bjoc.13.154
- with the facile elaboration of the spiropyran core, have made spiropyran–merocyanine systems a common motif in molecular switch and sensing applications [2]. In this respect, the difference in optical properties between colourless, non-fluorescent spiropyran and coloured, fluorescent merocyanine has
New synthetic strategies for xanthene-dye-appended cyclodextrins
Beilstein J. Org. Chem. 2016, 12, 537–548, doi:10.3762/bjoc.12.53

- acts as a complementary molecular switch to Rho-β-CD. While Rho-β-CD exhibits fluorescence at acidic pH, Flu-β-CD shows fluorescence at both neutral and alkaline pH. NMR characterization of Flu-β-CD The 1H NMR spectrum shown in Figure 7 is a typical spectrum of an asymmetric cyclodextrin. The sharp and
Synthesis and characterization of a new photoinduced switchable β-cyclodextrin dimer
Beilstein J. Org. Chem. 2014, 10, 2874–2885, doi:10.3762/bjoc.10.304

- to the guest molecules [9][10]. These cyclodextrins linked by ester [11], thioether [12][13][14][15][16], urea [17][18][19], or triazole [20] moieties have been previously described. In addition, aromatic azobenzenes are excellent candidates as molecular switch linkers as they have two forms, namely
- shifted slightly to a shorter wavelength and is significantly less intense at 320 nm. Because the n–π* transition is possible in the cis isomer, this band increases in intensity [22][23]. A molecular switch is based on the light-induced, reversible transformation of chemical species between two molecular
Design and synthesis of quasi-diastereomeric molecules with unchanging central, regenerating axial and switchable helical chirality via cleavage and formation of Ni(II)–O and Ni(II)–N coordination bonds
Beilstein J. Org. Chem. 2012, 8, 1920–1928, doi:10.3762/bjoc.8.223
- diastereomeric transformations are considered as the most promising models for the development of molecular switches with nondestructive read out of the optical information [14][15][16][17][18]. Taking into account the issue of fatigue resistance or durability of a potential molecular switch, the newly emerging
Control over molecular motion using the cis–trans photoisomerization of the azo group
Beilstein J. Org. Chem. 2012, 8, 1071–1090, doi:10.3762/bjoc.8.119

- ]. On the other hand, the isomerization can also occur through a rotation mechanism [11][30], which involves a π→π* transition (S0→S2) (Figure 3). This mechanism is similar to that produced in the isomerization of stilbene [23]. Azobenzenes as molecular switches A molecular switch is a molecular system
- that allows mechanical movements to be carried out when the system is subjected to an external stimulus, such as light, resulting in conformational and environmental changes of the switch. The basis of a molecular switch is the reversible transformation of chemical species caused by light between two
- condition for a molecule to behave as a switch is the existence of two different and stable isomeric forms that interconvert when an external stimulus is applied to it. The most important requirements for a molecule to behave as a molecular switch are the following [31][32][33][34][35]: The transformation
The effect of the formyl group position upon asymmetric isomeric diarylethenes bearing a naphthalene moiety
Beilstein J. Org. Chem. 2012, 8, 1018–1026, doi:10.3762/bjoc.8.114
- cytotoxicity [32]. Piao et al. developed a multiresponsive fluorescent molecular switch containing terpyridine. This diarylethene can serve as a detector for metal-ion transmembrane transport [33]. Singer et al. explored a novel diarylethene with a 7-deazaadenosine, which led to new research in photochromic
Recent advances towards azobenzene-based light-driven real-time information-transmitting materials
Beilstein J. Org. Chem. 2012, 8, 1003–1017, doi:10.3762/bjoc.8.113

- few decades. The interconversion between the different states of the molecular switch can be performed by a great variety of environmental stimuli, which can be classified into three main groups: light energy, electrical energy and chemical energy (pH, solvent, the presence of a determined metal or
- the azobenzene core is one of the main factors that allows modulating the thermal relaxation rate of azo-dyes and, therefore, determines the response time of the photochromic molecular switch. The response time of the photochromic switch is a key feature in its overall performance. This parameter is
On the bromination of the dihydroazulene/vinylheptafulvene photo-/thermoswitch
Beilstein J. Org. Chem. 2012, 8, 958–966, doi:10.3762/bjoc.8.108
- , renders the system interesting as a light-controlled molecular switch in, for example, molecular electronics. Indeed, light-induced conductance switching was recently observed for a DHA derivative situated in a single-molecule junction [4]. For the further exploration of the DHA/VHF switch in this field
Diarylethene-modified nucleotides for switching optical properties in DNA
Beilstein J. Org. Chem. 2012, 8, 905–914, doi:10.3762/bjoc.8.103
- only is a structural change observed but a large change in polarity is yielded additionally [10][22]. It is expected that the ring-closed spiropyran form of this molecular switch does not insert into the base stack due to its twisted structure, but the open merocyanine form could intercalate based on
- its planarity and polarity. This assumption was experimentally verified by synthetic spiropyrans as noncovalent DNA and RNA binders [23][24][25]. As expected, ground-state interactions between the noncovalently bound molecular switch and the DNA bases were detected in the case of the merocyanine form
The importance of the rotor in hydrazone-based molecular switches
Beilstein J. Org. Chem. 2012, 8, 872–876, doi:10.3762/bjoc.8.98
- of a series of hydrazone-based systems having different functional groups as part of the rotor (R = COMe, CN, Me, H), was studied. The switching efficiency of these systems was compared to that of a hydrazone-based molecular switch (R = COOEt) whose E/Z isomerization is fully reversible. It was found
- than PPH-5, suggesting that PPH-1 is a more ideal system to be used as a molecular switch. This analysis is clearly in line with the acid switching experiments that show that PPH-1 can be fully switched, whereas PPH-5 cannot. Conclusion In summary, we have synthesized four hydrazone-based systems

































































































































































































































































































